
This new classification system of PEA has 3 potential benefits compared to the traditional ACLS 5 H’s and 5 T’s

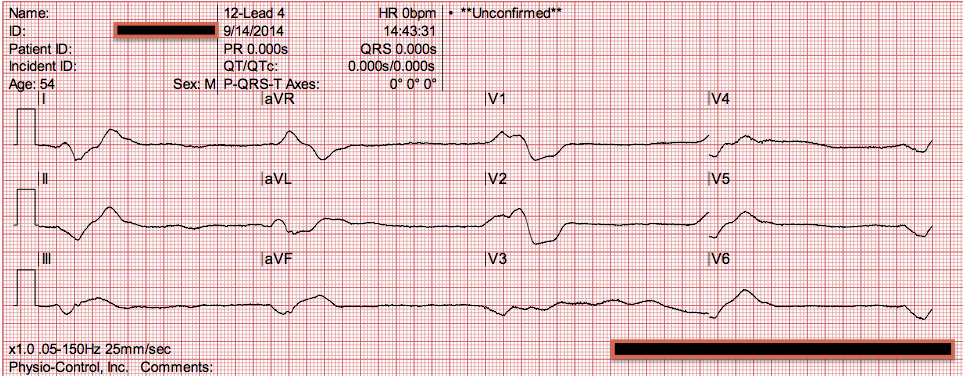
The morning that was planned for elective placement of hemodialysis catheter, she experienced ventricular tachycardia followed by pulseless electrical activity (PEA) arrest. She experienced accompanying symptoms of nausea and vomiting plans were made for initiation of hemodialysis to treat uremia. Diuretics were held, but renal function worsened to a BUN of 116 mg/dL and creatine of 4.65 mg/dL over 1 week. While maintaining negative fluid balance with oral furosemide, she experienced an acute kidney injury that was attributed to prerenal azotemia from over-diuresis.
Repeat echocardiogram demonstrated chronic diastolic dysfunction, RV failure, pulmonary hypertension (right ventricular systolic pressure, 78.7 mm Hg), and no pericardial effusion. With diuresis, her hemodynamics improved as did her renal function, and she was transferred to the hospital ward. Her ICU course was complicated by new onset atrial fibrillation for which she was started on apixaban 5 mg bid and rate control. She was admitted to the ICU and found to have new right ventricular (RV) failure for which she underwent aggressive diuresis, transiently requiring inotropic support. Laboratory results were remarkable for BUN 56 mg/dL, creatinine 2.41 mg/dL (from baseline creatinine 1.8 mg/dL,) and pro-brain natriuretic peptide >15,000 pg/mL chest radiography revealed bilateral pleural effusions. She had rales at both lung bases and significant pitting edema in the lower extremities. On examination, she was mentating well but was in mild distress. Her BP was 70/40 mm Hg oxygen saturation was 84% on room air respiratory rate was 30 breaths per minute, and heart rate was 73 beats per minute. A 73-year-old woman with a history of heart failure with preserved ejection fraction, severe pulmonary hypertension (World Health Organization class 2), and chronic kidney disease stage 3 presented to the ED after 2 days of weakness and shortness of breath.


 0 kommentar(er)
0 kommentar(er)
